COVID Update Briefs
These are some updates to the vaccination program that we have found over the last week. If you need more information please contact the AGPA Secretariat.
These are some updates to the vaccination program that we have found over the last week. If you need more information please contact the AGPA Secretariat.
Department of Health – Primary Health Care COVID-19 Response Teleconference 18 August 2021 – Meeting notes from Dr Maria Boulton
The Primary Care Implementation Group (PCIG) is an online meeting that includes most primary healthcare peak bodies. It is convened weekly by the Department of Health as part of the COVID-19 response. Dr Maria Boulton attends on behalf of the AGPA. Read more
Department of Health – Primary Health Care COVID-19 Response Teleconference 11 August 2021 – Meeting notes from Dr Maria Boulton
The Primary Care Implementation Group (PCIG) is an online meeting that includes most primary healthcare peak bodies. It is convened weekly by the Department of Health as part of the COVID-19 response. Dr Maria Boulton attends on behalf of the AGPA. Read more
Thursday 12 August: the ACT went into lock down with its first case of COVID-19 in over 12 months.
On Tuesday a Canberra respiratory clinic Principal commented:
A lot of hours and tired staff.
Friday 20 August: there are 94 active cases in the ACT, and more than 20,000 people are in quarantine.
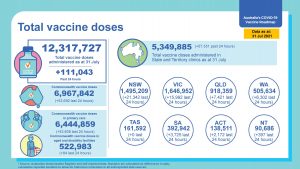
To date more than 13 M vaccine doses have been delivered, about 50% by GP
The full progress report can be found here

The DoH have provided strategic information regarding the Vaccine Program including Operation COVID Shield Plan, Doherty Modelling and Horizons Allocations:
The Department of Health have released a number of updates for Providers:
General Updates
Provider Bulletin 2 August 2021
Pfizer Updates
Pfizer Vaccine for Children Aged 12-15 Years

Pfizer Australia Pty Ltd
Level 15-18
151 Clarence Street
Sydney NSW 2000
26 July 2021
COMIRNATY (BNT162b2 [mRNA]) COVID-19 Vaccine: risk of myocarditis and pericarditis
Dear Healthcare Professional,
PFIZER in agreement with the Therapeutic Goods Administration would like to inform you of the following:
Summary
• Cases of myocarditis and pericarditis have been reported very rarely following vaccination with COMIRNATY.
• The cases primarily occurred within 14 days after vaccination, more often after the second dose and in younger men.
• Available data suggest that the course of myocarditis and pericarditis following vaccination is similar to the course of myocarditis and pericarditis in general.
• Healthcare professionals should be alert to the signs and symptoms of myocarditis and pericarditis.
• Healthcare professionals should advise vaccinated individuals to seek immediate medical attention should they experience chest pain, shortness of breath, or palpitations.
Background on the safety concern
COMIRNATY (BNT162b2[mRNA]) COVID-19 Vaccine has provisional approval for: Active immunisation to prevent coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2, in individuals 12 years of age and older. The use of this vaccine should be in accordance with official recommendations.
Myocarditis and pericarditis have been reported in association with COMIRNATY.
The Therapeutic Goods Administration has evaluated all available data and asked for the Product Information for COMIRNATY to be updated. Sections 4.4 ‘Special warnings and precautions for use’ and 4.8 ‘Adverse effects (Undesirable effects)’ have been updated.
The benefits of vaccination continue to outweigh any risks.
To 11 July 2021, approximately 3.7 million COMIRNATY doses have been administered, and the TGA has received 50 cases of suspected myocarditis and/or pericarditis relating to individuals vaccinated with COMIRNATY. (COVID-19 vaccine weekly safety report – 15-07-2021
https://www.tga.gov.au/periodic/covid-19-vaccine-weekly-safety-report-15-07-2021 )
Call for reporting
This vaccine is subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
Australian Distributors’ contact points
Pfizer Australia Pty Ltd
Level 17, 151 Clarence Street
Sydney NSW 2000
www.pfizer.com.au
Medical Information www.pfizermedinfo.com.au, or
Toll Free Number: 1800 675 229
Scott Williams
Vaccines Medical Director Australia, New Zealand and Korea
Department of Health – Primary Health Care COVID-19 Response Teleconference 4 August 2021 – Meeting notes from Dr Maria Boulton
The Primary Care Implementation Group (PCIG) is an online meeting that includes most primary healthcare peak bodies. It is convened weekly by the Department of Health as part of the COVID-19 response. Dr Maria Boulton attends on behalf of the AGPA. Read more
To date 12 M vaccine doses have been delivered, about 50% by GP
The full progress report can be found here
The Australian General Practice Alliance was formed in 2016 to represent the interests of GP practice owners. While the initial trigger for the formation of the AGPA was the attacks on General Practice by the Australian Government and big business pathology, our aim is to address the issues that face principal led General Practices.
Read more »
You can download a PDF application for Full Membership here.
You can Join and Pay Online here »