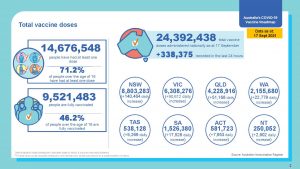
Over 24M Vaccine Doses Administered
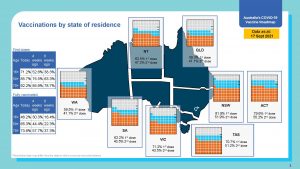
24.4 M vaccines have been administered at 17 September 2021
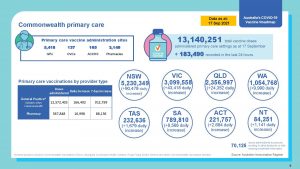
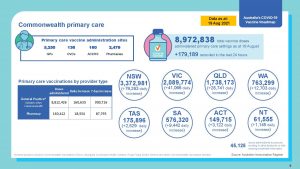
13.1 Vaccines have been administered by Commonwealth supported Primary Health Care 12.57M by GP
The full progress report to 17 September is here
24.4 M vaccines have been administered at 17 September 2021
13.1 Vaccines have been administered by Commonwealth supported Primary Health Care 12.57M by GP
The full progress report to 17 September is here



These are some updates to the vaccination program that we have found over the weeks. If you need more information please contact the AGPA Secretariat.
Eligibility Checker and Vaccine Clinic Finder updates: the Eligibility checker is to become a single page on the front of the Vaccine Clinic Finder. In future this will be referred to as the ‘Vaccine Clinic Finder’. This release is planned for Sunday 19 September 2021. Included in this release:
ATAGI Update 15 September: An update from the Australian Technical Advisory Group on Immunisation (ATAGI) following their weekly meeting on 15 September 2021 is available here.
Pfizer eligibility (14 September) :
Vaccination providers can administer Pfizer to all individuals aged 12 to 59.
Vaccination providers can also administer Pfizer to people aged 60 and over with:
• past history of cerebral venous sinus thrombosis (CVST)
• past history of Heparin-induced thrombocytopenia (HIT)
• past history of idiopathic splanchnic (mesenteric, portal, splenic) vein thrombosis
Over the last few weeks Dr Maria Boulton has asked a number of questions at the PCIG and directly to the task force. Some of these have now been answered.
A question and response are below:
We broadly support the engagement of students in primary care settings to assist with the COVID-19 response. There are a number of regulatory and employment arrangements, addition to whether such work should count towards professional experience placement (PEP) hours, that we encourage you to explore to enable such opportunities.
We further encourage you to liaise with all relevant jurisdictions on such a proposal, especially those that have not recognised students in their poisons legislation.
Over the last few weeks Dr Maria Boulton has asked a number of questions at the PCIG and directly to the task force. Some of these have now been answered.
A question and response are below:
Activities associated with the claiming of the COVID-19 MBS vaccine suitability assessment items may also be undertaken by a suitably qualified health professional who is working within their scope of practice.
Where a vaccination-related service is to be provided on behalf of a medical practitioner by a suitably qualified health professional, the medical practitioner must first consider whether the service is in accordance with accepted medical practice. This includes assessing if the suitably qualified health professional has the appropriate skills and training to administer the vaccine.
Important Note: Medical practitioners are prohibited from acting in the role of “suitably qualified health professional” on behalf of another medical practitioner. Under sub-section 3(17)(a) of the Health Insurance Act 1973, a service is taken to be rendered on behalf of a medical practitioner if, and only if, it is rendered by another person who is not a medical practitioner, and who provides the service, in accordance with accepted medical practice, under the supervision of the medical practitioner.
A suitably qualified health professional may assist a GP or Other Medical Practitioner (OMP) to provide a COVID-19 vaccine to a patient provided all of the following conditions are met:
Over the last few weeks Dr Maria Boulton has asked a number of questions at the PCIG and directly to the task force. Some of these have now been answered.
A question and response are below:
On 28 August 2021, the Australian Government announced details of the no fault COVID-19 Vaccine Claims Scheme, following extensive consultation with the peak medical, healthcare, business, and insurance sectors to ensure a comprehensive national Scheme.
The Scheme will provide people with quicker access to compensation where they have suffered an adverse event related to a Therapeutic Goods Administration (TGA) approved COVID-19 vaccination or its administration, irrespective of where the vaccination occurred.
If members are undertaking vaccinator roles using TGA-approved COVID-19 vaccines in private health care settings, this would still be considered within the scope of the Government’s COVID-19 Vaccines Program, and such practitioners’ patients would therefore be covered by the Scheme.
While side effects, or adverse events, from COVID-19 vaccinations can occur, most are mild and last no longer than a couple of days. Serious and life-threatening side effects are very rare. This Scheme provides a safety net to support those affected.
It also ensures that health professionals administering vaccines, including members, will be able to continue with their crucial role in the vaccine roll out with assurance that the claims scheme will offer them protection against time consuming litigation processes.
The Scheme will be administered by Services Australia and will provide people with a single front door to a simple and quick administrative process for compensation. The TGA will provide guidance on recognised adverse reactions as part of their established surveillance program.
The Scheme will cover the costs of injuries $5,000 and above due to a proven adverse reaction to a COVID-19 vaccine or its administration.
The cost of compensation payments under this Scheme will be fully funded by the Commonwealth and is designed to help the small number of people who unfortunately experience a moderate to significant adverse reaction to a COVID-19 vaccine.
The COVID-19 Vaccine Claims Scheme will be backdated to February 2021 and provide Australians with an alternative, administrative option to seek compensation, rather than a complex and costly court process.
At 2 September 20, 329 483 doses of COVID-19 Vaccine had been delivered.
10,649,670 had been delivered by General Practice – from 5,347 participating Practices
The full program summary is here
These are some updates to the vaccination program that we have found over the last week. If you need more information please contact the AGPA Secretariat.
Department of Health – Primary Health Care COVID-19 Response Teleconference 25 August 2021 – Meeting notes from Dr Maria Boulton
The Primary Care Implementation Group (PCIG) is an online meeting that includes most primary healthcare peak bodies. It is convened weekly by the Department of Health as part of the COVID-19 response. Dr Maria Boulton attends on behalf of the AGPA. Read more
Department of Health – Primary Health Care COVID-19 Response Teleconference 1 September 2021 – Meeting notes from Dr Maria Boulton
The Primary Care Implementation Group (PCIG) is an online meeting that includes most primary healthcare peak bodies. It is convened weekly by the Department of Health as part of the COVID-19 response. Dr Maria Boulton attends on behalf of the AGPA. Read more
Over 10.5 M Australians have had at least one dose of Vaccine.
GP has delivered 50% of the 16.5m doses delivered to date.
The full daily breakdown report is here

The Australian General Practice Alliance was formed in 2016 to represent the interests of GP practice owners. While the initial trigger for the formation of the AGPA was the attacks on General Practice by the Australian Government and big business pathology, our aim is to address the issues that face principal led General Practices.
Read more »
You can download a PDF application for Full Membership here.
You can Join and Pay Online here »